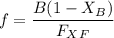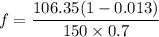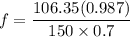Chemistry, 08.10.2020 01:01 joannakawata6
A distillation column is separating 150.0 kmol/h of a saturated liquid mixture that is 30.0 mol% methanol and 70.0 mol% water. The column operates at 1.0 atm pressure. Reflux ratio is 2.0, and reflux is returned as a saturated liquid. We desire a 97.0% recovery of methanol in the distillate and a methanol distillate mole fraction of 0.990. Find distillate ow rate D, bottoms flow rate B, methanol mole fraction in the bottoms xM, bot, and the fractional recovery of water in the bottoms.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Which is true of chemicals? a. things containing chemicals always cost a lot of money. b. chemicals are never dangerous. c. chemicals are in many substances in a home. d. chemicals are rarely found on earth.
Answers: 1

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1


Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
A distillation column is separating 150.0 kmol/h of a saturated liquid mixture that is 30.0 mol% met...
Questions

Biology, 30.04.2021 22:50



Social Studies, 30.04.2021 22:50


Mathematics, 30.04.2021 22:50


Arts, 30.04.2021 22:50




Mathematics, 30.04.2021 22:50




Mathematics, 30.04.2021 22:50


History, 30.04.2021 22:50

Social Studies, 30.04.2021 22:50

Mathematics, 30.04.2021 22:50

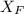 = 30%
= 30%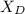 = 0.990
= 0.990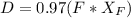
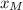 can be computed by using the formula:
can be computed by using the formula: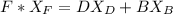
 )
)