
Chemistry, 08.10.2020 01:01 Jasminehenry123
answer the questions below, using lt (for is less than), gt (for is greater than), eq (for equal to), or mi (for more information) in the blanks provided. 1. the wavelength of the photon required to promote an electron in the hydrogen atom from the n = 1 to the n = 3 level is the wavelength of the photon required to promote an electron in the hydrogen atom from the n =1 to the n = 2 level. 2. the energy of a photon with a wavelength of 463 nm is the energy of a photon whose wavelength is 722 nm. 3. in order to promote an electron to go to a higher energy level, light with a wavelength that is 400 nm is required.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

You know the right answer?
answer the questions below, using lt (for is less than), gt (for is greater than), eq (for equal to)...
Questions

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Physics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Biology, 13.09.2020 14:01

History, 13.09.2020 14:01

English, 13.09.2020 14:01

Physics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

History, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

English, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

Mathematics, 13.09.2020 14:01

English, 13.09.2020 14:01

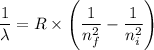
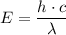
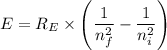
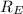 = -2.178 × 10⁻¹⁸ J
= -2.178 × 10⁻¹⁸ J 

