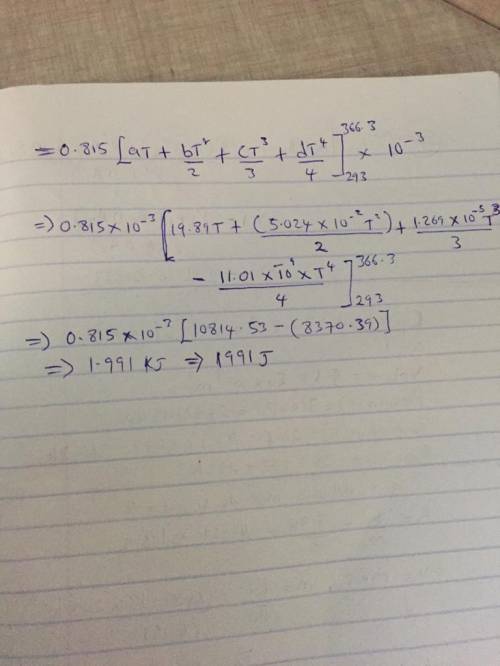The specific heats at constant pressure of some common gases are provided as a thirdorder polynomial: �;<<< = � + �� + ��C + ��E, with units J/(mol K). For methane (CH4) the coefficients are � = 19.89, � = 5.024 × 10NC, � = 1.269 × 10NO, � = −11.01 × 10NQ. At a production facility, the gas in a 50-liter tank is compressed to 3,600 psi (gage) during which time the temperature rises to 200°F. How much heat in J is given off as the gas cools to room temperature? Suppose the compressed gas is helium, how much heat would be given off in this case?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
The specific heats at constant pressure of some common gases are provided as a thirdorder polynomial...
Questions

English, 21.09.2019 06:10

English, 21.09.2019 06:10

Social Studies, 21.09.2019 06:10

Computers and Technology, 21.09.2019 06:10


Mathematics, 21.09.2019 06:10

Arts, 21.09.2019 06:10


Mathematics, 21.09.2019 06:10

History, 21.09.2019 06:10



Health, 21.09.2019 06:10

Mathematics, 21.09.2019 06:10


Mathematics, 21.09.2019 06:10



Business, 21.09.2019 06:10

Geography, 21.09.2019 06:10