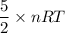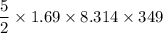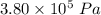A tank contains 120.0 g chlorine gas (Cl2), which is at temperature 76.0°C and absolute pressure 5.70 ✕ 105 Pa. The temperature of the air outside the tank is 19.0°C. The molar mass of Cl2 is 70.9 g/mol. (a) What is the volume of the tank (in m3)? m3 (b) What is the internal energy of the gas (in J)? J (c) What is the work done by the gas (in J) if the temperature and pressure inside the tank drop to 31.0°C and 3.80 ✕ 105 Pa, respectively, due to a leak? (Assume that the air outside the tank can be treated as a vacuum.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

You know the right answer?
A tank contains 120.0 g chlorine gas (Cl2), which is at temperature 76.0°C and absolute pressure 5.7...
Questions


History, 11.11.2020 23:00



Mathematics, 11.11.2020 23:00

Mathematics, 11.11.2020 23:00









Mathematics, 11.11.2020 23:10



English, 11.11.2020 23:10


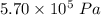
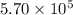
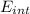 =
=