
How many σ and π bonds are in this molecule? A chain of five carbon atoms. There is a double bond between the first and second carbon atoms and a triple bond between the fourth the fifth carbon atoms. There are single bonds between the remaining carbon atoms. There are two hydrogen atoms bonded to the first carbon atom through single bonds, and a single hydrogen atom bonded to both the second and fifth carbon atoms through single bonds. There is an oxygen atom bonded to the third carbon atom through a double bond.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
How many σ and π bonds are in this molecule? A chain of five carbon atoms. There is a double bond be...
Questions


History, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30


Spanish, 04.07.2019 16:30

English, 04.07.2019 16:30



Spanish, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30


Mathematics, 04.07.2019 16:30

Computers and Technology, 04.07.2019 16:30

Mathematics, 04.07.2019 16:30


English, 04.07.2019 16:30


Mathematics, 04.07.2019 16:30

History, 04.07.2019 16:30

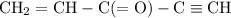 .
.

