Chemistry, 08.10.2020 07:01 musiclyhollywoodbabo
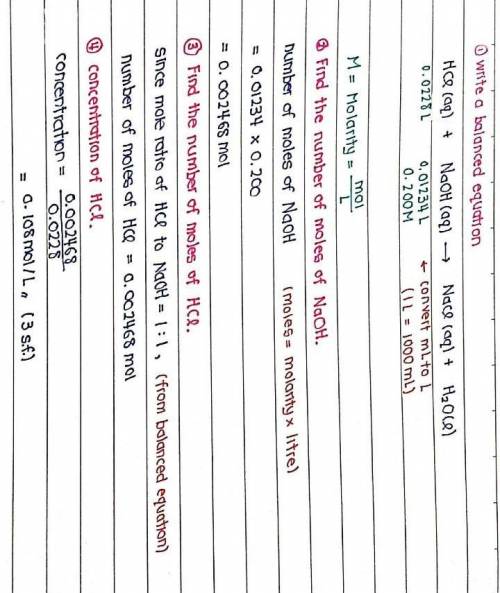
The titration of 22.80 mL of HCl solution of unknown concentration requires 12.34 mL of a 0.200 M NaOH solution to reach the equivalence point. What is the concentration of the unknown HCl solution in M? Express your answer in moles per liter to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1
You know the right answer?
The titration of 22.80 mL of HCl solution of unknown concentration requires 12.34 mL of a 0.200 M Na...
Questions


Arts, 29.01.2020 14:50


Computers and Technology, 29.01.2020 14:50



History, 29.01.2020 14:50

Computers and Technology, 29.01.2020 14:50


Mathematics, 29.01.2020 14:50

Mathematics, 29.01.2020 14:50



Mathematics, 29.01.2020 14:50

Mathematics, 29.01.2020 14:50