
Chemistry, 08.10.2020 08:01 michaelgold1
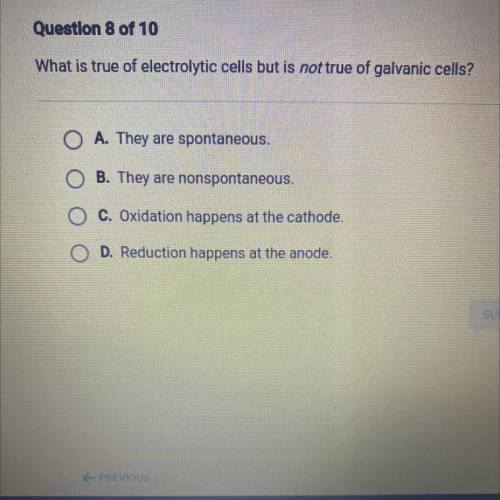
What is true of electrolytic cells but is not true of galvanic cells?
O A. They are spontaneous.
O B. They are nonspontaneous.
O C. Oxidation happens at the cathode.
O D. Reduction happens at the anode.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
What is true of electrolytic cells but is not true of galvanic cells?
O A. They are spontaneous.
Questions

Mathematics, 03.07.2019 17:30

Mathematics, 03.07.2019 17:30

English, 03.07.2019 17:30

Mathematics, 03.07.2019 17:30

Mathematics, 03.07.2019 17:30

Mathematics, 03.07.2019 17:30



Mathematics, 03.07.2019 17:30

Mathematics, 03.07.2019 17:30

English, 03.07.2019 17:30


Mathematics, 03.07.2019 17:30



Mathematics, 03.07.2019 17:30

Mathematics, 03.07.2019 17:30

English, 03.07.2019 17:30

Mathematics, 03.07.2019 17:30



