Mercury(II) oxide decomposes to form mercury and oxygen, like this:
2HgO(s)Hg(l) + O2(g)
At...

Chemistry, 08.10.2020 14:01 PresleyPie2700
Mercury(II) oxide decomposes to form mercury and oxygen, like this:
2HgO(s)Hg(l) + O2(g)
At a certain temperature, a chemist finds that a reaction vessel containing a mixture of mercury(II) oxide, mercury, and oxygen at equilibrium has the following composition:
compound amount
HgO 24.0g
Hg 23.6g
O2 22.7g
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Questions


Mathematics, 26.07.2021 14:50

Social Studies, 26.07.2021 14:50



French, 26.07.2021 14:50

Mathematics, 26.07.2021 14:50



Business, 26.07.2021 14:50


Mathematics, 26.07.2021 14:50

Business, 26.07.2021 14:50

Mathematics, 26.07.2021 14:50

English, 26.07.2021 14:50

Social Studies, 26.07.2021 14:50


Mathematics, 26.07.2021 14:50


Biology, 26.07.2021 14:50

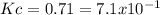
![Kc=[O_2]](/tpl/images/0794/3421/af0f4.png)
![[O_2]=\frac{22.7g*\frac{1mol}{32g} }{1L} =0.709M](/tpl/images/0794/3421/9d34c.png)


