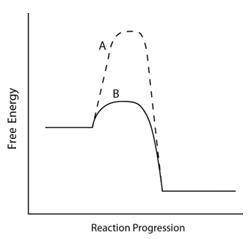
Consider the energy diagram below.
Which line indicates a higher reaction rate?
A because it...

Chemistry, 09.10.2020 19:01 gaberamos973
Consider the energy diagram below.
Which line indicates a higher reaction rate?
A because it has a lower activation energy.
B because it has a lower activation energy.
A because its (angle)Grxn is much lower.
B because its (angle)Grxn is much lower.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
Questions

Computers and Technology, 24.09.2021 18:10


Physics, 24.09.2021 18:10

Mathematics, 24.09.2021 18:10

Mathematics, 24.09.2021 18:10



Mathematics, 24.09.2021 18:10




Mathematics, 24.09.2021 18:10



Chemistry, 24.09.2021 18:10


History, 24.09.2021 18:10

History, 24.09.2021 18:10

Physics, 24.09.2021 18:10



