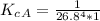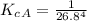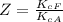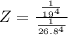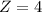Chemistry, 09.10.2020 23:01 andybiersack154
The protein hemoglobin (Hb) transports O2 in mammalian blood. Each Hb can bind 4O2 molecules. The equilibrium constant for the O2-binding reaction is higher in fetal hemoglobin than in adult hemoglobin. In discussing protein oxygen-binding capacity, biochemists use a measure called the P50 value, defined as the partial pressure of oxygen at which 50% of the protein is saturated. Fetal hemoglobin has a P50 value of 19 torr, and adult hemoglobin has a P50 value of 26.8 torr. Use these data to estimate how much larger Kc is for the aqueous reaction 4O2(g)+Hb(aq)→[Hb(O2)4(aq)].

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1


Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
The protein hemoglobin (Hb) transports O2 in mammalian blood. Each Hb can bind 4O2 molecules. The eq...
Questions

History, 07.04.2021 21:10



History, 07.04.2021 21:10


Mathematics, 07.04.2021 21:10

Mathematics, 07.04.2021 21:10

Mathematics, 07.04.2021 21:10

Mathematics, 07.04.2021 21:10

Chemistry, 07.04.2021 21:10

Biology, 07.04.2021 21:10

History, 07.04.2021 21:10

Mathematics, 07.04.2021 21:10

Mathematics, 07.04.2021 21:10

Health, 07.04.2021 21:10

Mathematics, 07.04.2021 21:10

Mathematics, 07.04.2021 21:10



World Languages, 07.04.2021 21:10

![[P_{O_2}]_F = 19 torr](/tpl/images/0795/3120/d5cf5.png)
![[P_{O_2}]_A = 26.8 torr](/tpl/images/0795/3120/e335c.png)
![4O_2_{(g)}+Hb_{(aq)}\to [Hb(O_2)_4_{{(aq)}}]](/tpl/images/0795/3120/45f03.png)
![K_c_F = \frac{[P_{[Hb(O_2)_4}]}{ [P_{O_2}]_F^4 * [P_{Hb}]}](/tpl/images/0795/3120/f4794.png)
![[P_{[Hb(O_2)_4}] [\tex] and [P_{Hb}] will be 1 because both substances are aqueousSo [tex]K_c_F = \frac{1}{ 19^4 *1 }](/tpl/images/0795/3120/59557.png)
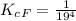
![K_c_A = \frac{[P_{[Hb(O_2)_4}]}{ [P_{O_2}]_A^4 * [P_{Hb}]}](/tpl/images/0795/3120/10577.png)