
Chemistry, 10.10.2020 22:01 mrashrafkotkaat
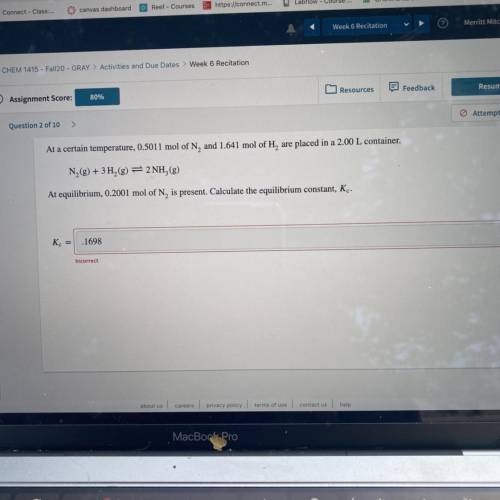
At a certain temperature, 0.5011 mol of N, and 1.641 mol of H, are placed in a 2.00 L container.
N2(g) + 3H2(g) = 2NH,(g)
At equilibrium, 0.2001 mol of N, is present. Calculate the equilibrium constant, Kc.
K =
.1698
Incorrect
about us
careers
privacy policy
terms of use
contact Us
help

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
You know the right answer?
At a certain temperature, 0.5011 mol of N, and 1.641 mol of H, are placed in a 2.00 L container.
N2...
Questions

Mathematics, 18.10.2019 00:30

Mathematics, 18.10.2019 00:30

Mathematics, 18.10.2019 00:30

Mathematics, 18.10.2019 00:30



Mathematics, 18.10.2019 00:30





English, 18.10.2019 00:30

Business, 18.10.2019 00:30

Mathematics, 18.10.2019 00:30


Mathematics, 18.10.2019 00:30


Mathematics, 18.10.2019 00:30

Biology, 18.10.2019 00:30



