check all that apply.

Why are most organic compounds nonconducting and insoluble in water?
check all that apply.
most organic compounds don't dissolve in water because they are polar.
most organic compounds don't dissolve in water because they are nonpolar.
they don't conduct electricity because they are ionic, not covalent.
they don't conduct electricity because they are covalent, not ionic.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

You know the right answer?
Why are most organic compounds nonconducting and insoluble in water?
check all that apply.
check all that apply.
Questions


English, 22.06.2019 22:40


English, 22.06.2019 22:40


Chemistry, 22.06.2019 22:40




Geography, 22.06.2019 22:40



English, 22.06.2019 22:40


Biology, 22.06.2019 22:40





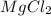 etc that are also polar in nature.
etc that are also polar in nature.

