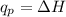Identify each enthalpy change by name and classify each change as exothermic or endothermic.
1. 1 mol nacl (s) à 1 mol nacl(l)
2. c4h10 o(l) à c4h10o (g)
3. h2o(g) à h2o (l)
4. 1 mol nacl(s) + 3.88 kj/molà 1 mol nacl(aq)
a molar heat of solution; endothermic
b molar heat of vaporization; endothermic
c molar heat of fusion; endothermic
d molar heat of condensation; exothermic

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Identify each enthalpy change by name and classify each change as exothermic or endothermic.
Questions

Mathematics, 14.11.2019 05:31




Mathematics, 14.11.2019 05:31





History, 14.11.2019 05:31

Mathematics, 14.11.2019 05:31


Mathematics, 14.11.2019 05:31

Mathematics, 14.11.2019 05:31




Mathematics, 14.11.2019 05:31