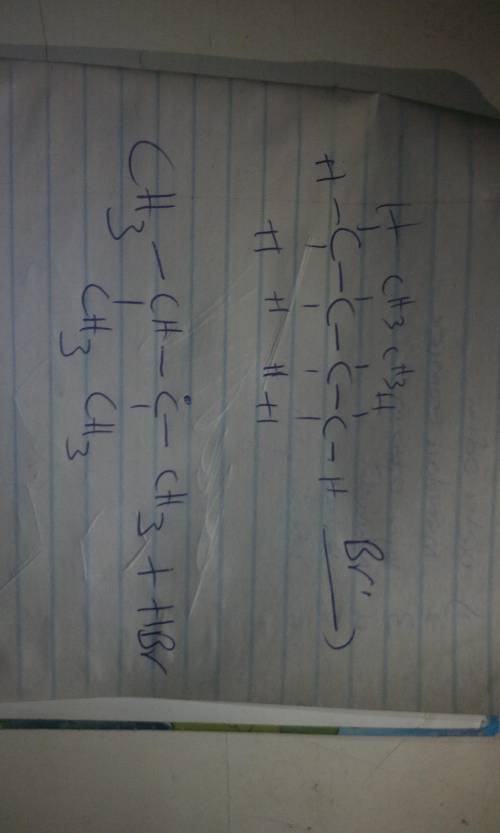In the first propagation step of the bromination of 2,3-dimethylbutane, the newly formed Br radicals react with the molecules of 2,3-dimethylbutane. Give the major products formed by the first propagation step including both organic and inorganic products. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. Include all free radicals by right-clicking on an atom on the canvas and then using the Atom properties to select the monovalent radical.

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1

Chemistry, 23.06.2019 11:30
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3

You know the right answer?
In the first propagation step of the bromination of 2,3-dimethylbutane, the newly formed Br radicals...
Questions



Mathematics, 18.03.2021 02:00

History, 18.03.2021 02:00


Health, 18.03.2021 02:00



English, 18.03.2021 02:00


Mathematics, 18.03.2021 02:00


Mathematics, 18.03.2021 02:00


English, 18.03.2021 02:00


Mathematics, 18.03.2021 02:00

Biology, 18.03.2021 02:00

Engineering, 18.03.2021 02:00