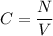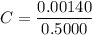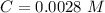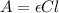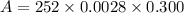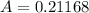Chemistry, 12.10.2020 14:01 anferneebcoleman
The molar absorptivity of a compound at 500 nm wavelength is 252 M-1cm-1. Suppose one prepares a solution by dissolving 0.00140 moles of a solute in enough water to make a 500.0 mL solution. What would be the absorbance in a 3 .00 mm pathlength cell?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3


Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
The molar absorptivity of a compound at 500 nm wavelength is 252 M-1cm-1. Suppose one prepares a sol...
Questions

Health, 14.09.2020 18:01

Social Studies, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Physics, 14.09.2020 18:01

English, 14.09.2020 18:01

Geography, 14.09.2020 18:01

Geography, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

History, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Biology, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01

Mathematics, 14.09.2020 18:01