
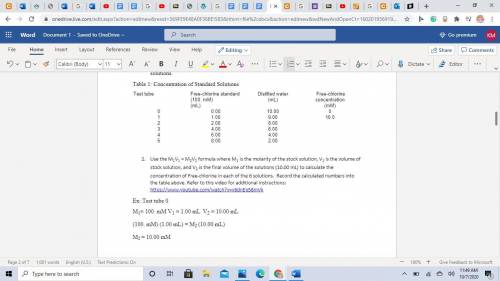
A 100mM/L solution of Free-chlorine stock solution was used to make up 6 standard solutions (known concentration). See the table below with gives the test tube number, the amount of stock solution used and the amount of distilled water used to make up these solutions.
Table 1: Concentration of Standard Solutions
Test tube
Free-chlorine standard (100. mM)
(mL)
Distilled water
(mL)
Free-chlorine concentration
(mM)
0
0.00
10.00
0
1
1.00
9.00
10.0
2
2.00
8.00
3
4.00
6.00
4
6.00
4.00
5
8.00
2.00
Use the M1V1 = M2V2 formula where M1 is the molarity of the stock solution, V1 is the volume of stock solution, and V2 is the final volume of the solutions (10.00 mL) to calculate the concentration of Free-chlorine in each of the 6 solutions. Record the calculated numbers into the table above. Ex: Test tube 0
M1= 100. mM V1 = 1.00 mL V2 = 10.00 mL
(100. mM) (1.00 mL) = M2 (10.00 mL)
M2 = 10.00 mM
(there's also a screenshot of the problem if that's easier)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

You know the right answer?
A 100mM/L solution of Free-chlorine stock solution was used to make up 6 standard solutions (known c...
Questions

History, 08.11.2020 22:30




Biology, 08.11.2020 22:30

Chemistry, 08.11.2020 22:30

Computers and Technology, 08.11.2020 22:30

Mathematics, 08.11.2020 22:30

Arts, 08.11.2020 22:30

Mathematics, 08.11.2020 22:30


Mathematics, 08.11.2020 22:30



Arts, 08.11.2020 22:30


Chemistry, 08.11.2020 22:30


Mathematics, 08.11.2020 22:30

Mathematics, 08.11.2020 22:30



