
Chemistry, 13.10.2020 01:01 sidneybrsimmons
The second-order rate constant for the following gas-phase reaction is 0.048 1/MLaTeX: \cdot⋅s. We start with 0.1 mol C2F4 in a 2.31 liter container, with no C4F8 initially present. C2F4 LaTeX: \longrightarrow⟶ 1/2 C4F8 What will be the concentration of C4F8 after 2.1 hours?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
The second-order rate constant for the following gas-phase reaction is 0.048 1/MLaTeX: \cdot⋅s. We s...
Questions

Mathematics, 01.12.2020 06:40



Geography, 01.12.2020 06:40

Mathematics, 01.12.2020 06:40


History, 01.12.2020 06:40


Chemistry, 01.12.2020 06:40

Mathematics, 01.12.2020 06:40

Mathematics, 01.12.2020 06:40

Mathematics, 01.12.2020 06:40

History, 01.12.2020 06:40


History, 01.12.2020 06:40

Spanish, 01.12.2020 06:40

Mathematics, 01.12.2020 06:40



![[A]=0.0026M](/tpl/images/0801/6543/fc05e.png)
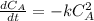
![\frac{1}{[A]}= \frac{1}{[A_0]} +kt](/tpl/images/0801/6543/17716.png)
![[A]_0=\frac{0.1mol}{2.31L} =0.043M](/tpl/images/0801/6543/8622a.png)
![\frac{1}{[A]}= \frac{1}{0.043M} +\frac{0.048}{M*s} *\frac{3600s}{1h}*2.1h\\\\\frac{1}{[A]}=\frac{386.1}{M}](/tpl/images/0801/6543/e0dcc.png)
![[A]=\frac{M}{386.1} =0.0026M](/tpl/images/0801/6543/394b0.png)


