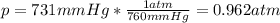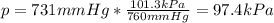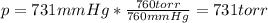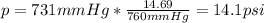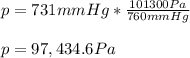Chemistry, 13.10.2020 01:01 itsgiovanna
Caed for this question.
A student reads a barometer in the laboratory and finds the prevailing atmospheric pressure to be 731 mm Hg. Express this
pressure in atmospheres, kilopascals, torrs, pounds per square inch, and pascals.
Hint: 1 atm
101.3 kPa = 760 torr = 760 mm Hg = 14.69 psi = 1.013*10Pa
mm Hg
atm
kPa
torr
psi
Pa
731
Submit Answer
Retry Entire Group
8 more group attempts remaining

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Caed for this question.
A student reads a barometer in the laboratory and finds the prevailing atmo...
Questions




Mathematics, 08.10.2019 00:30




Advanced Placement (AP), 08.10.2019 00:30




English, 08.10.2019 00:30

Social Studies, 08.10.2019 00:30

Geography, 08.10.2019 00:30