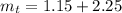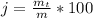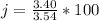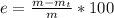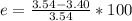A student was asked to determine the percentage of each component in a mixture of silver nitrate, AgNO3 and magnesium hydroxide, Mg(OH)2. The mass of the sample used was 3.54 g. The student extracted AgNO3 from the mixture with water and separated the insoluble Mg(OH)2 from the solution by filtration. After evaporating the filtrate to dryness, the student recovered and dried the AgNO3, and found that it weighed 1.15 g. After drying the recovered Mg(OH)2, a mass of 2.25 g was recorded. On the basis of the mass of the sample used:
1. Calculate the % AgNO3 in the mixture,
2, Calculate the % Mg(OH)2 in the mixture.
3. Calculate the total mass of the AgNO3 and Mg(OH)2 recovered.
4. Calculate the % recovery of the components, using the total mass of the substances recovered.
5. Calculate the % error for the separation of the components of the mixture.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Microtubular fibers that assist in the movement of chromosomes during nuclear division in conjunction with proteins, makes up the small and large organelle pieces that assemble prior to translation three-base nucleotide sequence that can complementarily pair with the functional transcript, allowing a particular material to be brought to a ribosome particular time in the cell cycle when the cell’s systems determine if the cellular conditions are appropriate to continue through the cycle time in the cell’s cycle when proteins are made and organelles are duplicated enzyme that allows proper nucleotide bonding during transcription specific dna sequence which will initiate gene transcription division of the cell’s cytoplasm specific bond that forms between two amino acids when a carboxyl group binds to a amino group three-base sequence that does not code for a particular amino acid a paired organelle which facilitates the formation of movement microtubules time in the cell’s cycle when the microtubular structures exert an equal pressure on the cell’s genetic material
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
A student was asked to determine the percentage of each component in a mixture of silver nitrate, Ag...
Questions

History, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 04:01

Mathematics, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

Biology, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

Mathematics, 13.09.2020 05:01

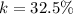
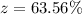
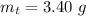
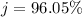
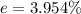
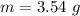
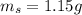
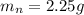
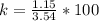
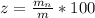
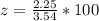
![[tex]m_t = m_s +m_n](/tpl/images/0802/4286/6b7c5.png)