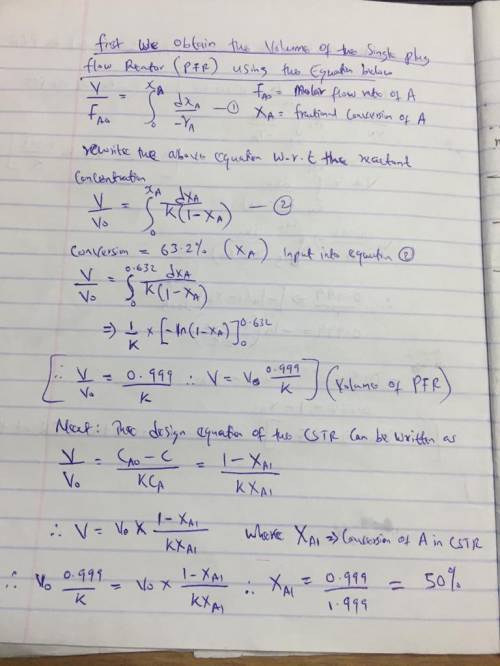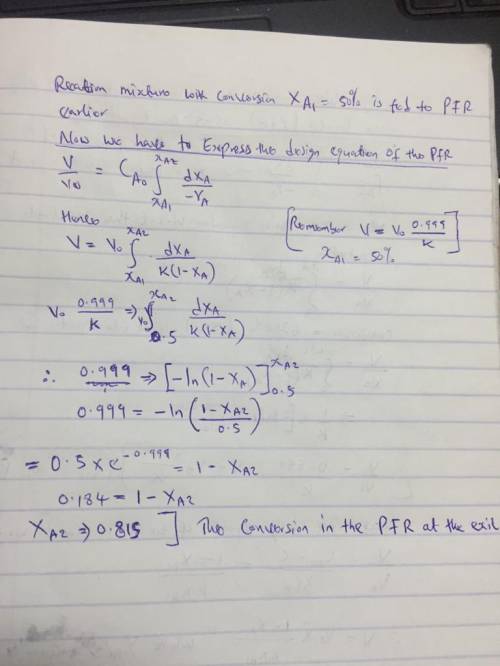The elementary gas-phase reaction
A → B
takes place isobarically and isothermally in a...

Chemistry, 13.10.2020 05:01 taekookie01
The elementary gas-phase reaction
A → B
takes place isobarically and isothermally in a PFR where 63.2% conversion is achieved. The feed is pure A. It is proposed to put a CSTR of equal volume upstream of the PFR. Based on the entering molar flow rate to A to the first reactor, what will be the intermediate from the CSTR, X1, and exit conversion from the PFR, X2, based on the feed to first reactor?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Questions


Mathematics, 13.11.2019 00:31




Biology, 13.11.2019 00:31

History, 13.11.2019 00:31


Mathematics, 13.11.2019 00:31


Spanish, 13.11.2019 00:31

Mathematics, 13.11.2019 00:31

Computers and Technology, 13.11.2019 00:31



English, 13.11.2019 00:31




Mathematics, 13.11.2019 00:31