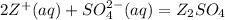Chemistry, 13.10.2020 05:01 brittanyowusu1820
A chem 1515 student was given a solution of the cations X3 , Y2 , and Z in the form of nitrates. Through a series of tests, she obtained the following information:
Reagent X3 Y2 Z
Na2SO4 No Reaction Forms white ppt. Brown ppt. forms
NH4OH No Reaction No Reaction ppt. Dissolves
HCl No Reaction No Reaction White ppt. forms
Na3PO4 White ppt. forms No Reaction No Reaction
The reaction between Z (aq) and Na2SO4 forms a brown ppt.
Required:
Write a balanced chemical equation for all of the reactions which result in the formation of a precipitate

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
A chem 1515 student was given a solution of the cations X3 , Y2 , and Z in the form of nitrates. Thr...
Questions

Mathematics, 01.11.2019 12:31

History, 01.11.2019 12:31


Physics, 01.11.2019 12:31

Mathematics, 01.11.2019 12:31


Mathematics, 01.11.2019 12:31


Social Studies, 01.11.2019 12:31




Mathematics, 01.11.2019 12:31




Biology, 01.11.2019 12:31


Health, 01.11.2019 12:31