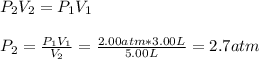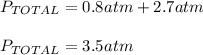Chemistry, 13.10.2020 20:01 therronvictorjr
What will happen when the valve between a 2.00-L bulb, in which the gas pressure is 2.00 atm, and a 3.00-L bulb, in which the gas pressure is 4.50 atm, is opened? Assume the temperature remains constant. (For this question, label each statement as True or False and give an explanation/justification for each of your answers to receive credit. No credit is given for only True or False answers with no adequate justification. To receive credit you must provide justification. You may have to give numerical answers followed by verbal explanations. You can use any reasoning that you see fit but be as thorough as possible.)
The two gases will mix and react.
The two gases will remain separate and will not mix.
The two gases will occupy a volume of 5.0 L and the final pressure in the two bulbs will be 6.50 atm.
The two gases will occupy a volume of 5.0 L and the final pressure in the two bulbs will be 3.50 atm.
The two gases will occupy a volume of 5.0 L and the final pressure in the two bulbs will be 3.25 atm.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1
You know the right answer?
What will happen when the valve between a 2.00-L bulb, in which the gas pressure is 2.00 atm, and a...
Questions

Mathematics, 28.10.2019 05:31

Biology, 28.10.2019 05:31

Mathematics, 28.10.2019 05:31


French, 28.10.2019 05:31

Physics, 28.10.2019 05:31

Physics, 28.10.2019 05:31

Mathematics, 28.10.2019 05:31


Chemistry, 28.10.2019 05:31

Mathematics, 28.10.2019 05:31






Geography, 28.10.2019 05:31


Mathematics, 28.10.2019 05:31

Mathematics, 28.10.2019 05:31