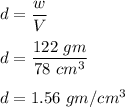Chemistry, 14.10.2020 02:01 thomasbarbusca15
You would like to find the density of an unusually shaped piece of jewelry. Since the piece of jewelry has an irregular shape, you need to use displacement to calculate the volume. In a 300 ml beaker you pour 200 ml. Of water. When you place the piece of jewelry in the beaker, the water level rises to 278 ml. Upon placing the object on the triple beam balance, you find that it weighs 122 grams. Given this information, calculate the density. SHOW YOUR WORK

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
You would like to find the density of an unusually shaped piece of jewelry. Since the piece of jewel...
Questions







Social Studies, 24.04.2021 05:30

Mathematics, 24.04.2021 05:30

Mathematics, 24.04.2021 05:30

Arts, 24.04.2021 05:30



Spanish, 24.04.2021 05:30

Biology, 24.04.2021 05:30

Arts, 24.04.2021 05:30

Mathematics, 24.04.2021 05:30



Chemistry, 24.04.2021 05:30