
Chemistry, 15.10.2020 04:01 corbinfisher
If you had 1.37 x 1022 atoms of lead (Pb), what would the mass of that sample be?
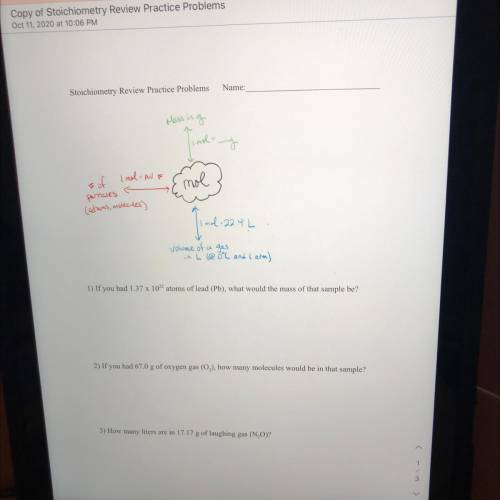

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
If you had 1.37 x 1022 atoms of lead (Pb), what would the mass of that sample be?
...
...
Questions







Social Studies, 07.05.2020 14:57


Computers and Technology, 07.05.2020 14:57




Computers and Technology, 07.05.2020 14:57

Chemistry, 07.05.2020 14:57




History, 07.05.2020 14:57





