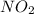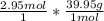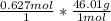Calculate the mass, in grams, of 2.95 mol of argon, Ar.
mass of Ar:
Calculate the mass, in gr...

Chemistry, 15.10.2020 06:01 billy12008
Calculate the mass, in grams, of 2.95 mol of argon, Ar.
mass of Ar:
Calculate the mass, in grams, of 0.627 mol of nitrogen dioxide, NOZ.
mass of NO2:

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Questions

Mathematics, 01.10.2019 05:30

History, 01.10.2019 05:30




Chemistry, 01.10.2019 05:30

Mathematics, 01.10.2019 05:30






Social Studies, 01.10.2019 05:30

Mathematics, 01.10.2019 05:30




Mathematics, 01.10.2019 05:30