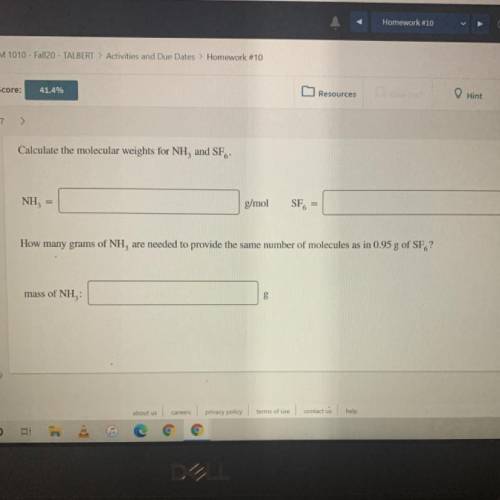
Calculate the molecular weights for NH, and SF.
NH, =
g/mol
SF, =
How many grams...

Chemistry, 15.10.2020 06:01 jilianfirmanp0hz9l
Calculate the molecular weights for NH, and SF.
NH, =
g/mol
SF, =
How many grams of NH, are needed to provide the same number of molecules as in 0.95 g of SF.?
mass of NH3:
g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Questions

Computers and Technology, 18.10.2019 19:50


Mathematics, 18.10.2019 19:50

Mathematics, 18.10.2019 19:50

Computers and Technology, 18.10.2019 19:50

English, 18.10.2019 19:50


Spanish, 18.10.2019 19:50


History, 18.10.2019 19:50

Social Studies, 18.10.2019 19:50

Mathematics, 18.10.2019 19:50

Mathematics, 18.10.2019 19:50

Computers and Technology, 18.10.2019 19:50




Spanish, 18.10.2019 19:50

English, 18.10.2019 19:50

Mathematics, 18.10.2019 19:50



