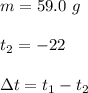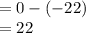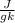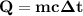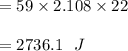At 1 atm, how much energy is required to heat 59.0 g H2O(s) at −22.0 ∘C to H2O(g) at 141.0 ∘C? STRATEGY: Calculate the energy needed for each temperature change or phase change individually. a. The energy needed to heat 59.0 g H2O(s) from −22.0 ∘C to its melting point. b. The energy needed to melt 59.0 g H2O(s) at its melting point. c. The energy needed to heat 59.0 g H2O(l) from the melting point to the boiling point. d. The energy needed to boil 59.0 g H2O(l) at its boiling point. e. The energy needed to heat 59.0 g H2O(g) from the boiling point to 141.0 ∘C. Sum the energies from each step and convert to kilojoules. Step 1. a. Going from −22.0 ∘C to the melting point of H2O (0 ∘C) is a temperature change of 22.0 ∘C. How much energy is needed to heat 59.0 g H2O(s) by 22.0 ∘C if its specific heat is 2.087 J/(g× ∘C)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
At 1 atm, how much energy is required to heat 59.0 g H2O(s) at −22.0 ∘C to H2O(g) at 141.0 ∘C? STRAT...
Questions

History, 05.05.2020 13:43

Social Studies, 05.05.2020 13:43



Advanced Placement (AP), 05.05.2020 13:43



History, 05.05.2020 13:43



Mathematics, 05.05.2020 13:43

Mathematics, 05.05.2020 13:43

Mathematics, 05.05.2020 13:43




Chemistry, 05.05.2020 13:43

Mathematics, 05.05.2020 13:43