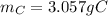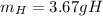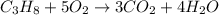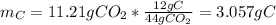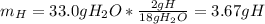Help I need to show work!
Question: The result of a reaction of propane and air gave 11.21 g CO2 and 33.0 grams water according
to the following equation: C3H3 + 5 02 + 3 CO2 + 4 H20
a) What type of reaction (name reaction) is this?
b. How many grams of carbon were produced?
C. How many grams of hydrogen were produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Help I need to show work!
Question: The result of a reaction of propane and air gave 11.21 g CO2 an...
Questions


Social Studies, 25.03.2020 05:51

Mathematics, 25.03.2020 05:51


Mathematics, 25.03.2020 05:51

Mathematics, 25.03.2020 05:51


History, 25.03.2020 05:51

English, 25.03.2020 05:51





Mathematics, 25.03.2020 05:51


Mathematics, 25.03.2020 05:51