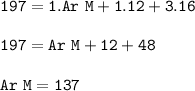Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

Chemistry, 23.06.2019 19:10
Which us an example of the practical pursuit of alchemy? a. linking spiritual characteristics with material substances b. forming perfect substances c. developing metalworking technique d. transforming base metals
Answers: 2
You know the right answer?
The relative formula mass (M) of a Group 2 metal carbonate is 197
Relative atomic masses (Ar): C =...
Questions



Business, 07.01.2020 22:31

History, 07.01.2020 22:31

Computers and Technology, 07.01.2020 22:31

Social Studies, 07.01.2020 22:31


Biology, 07.01.2020 22:31

History, 07.01.2020 22:31


History, 07.01.2020 22:31

Mathematics, 07.01.2020 22:31






Mathematics, 07.01.2020 22:31


Mathematics, 07.01.2020 22:31