
Chemistry, 16.10.2020 20:01 hargunk329
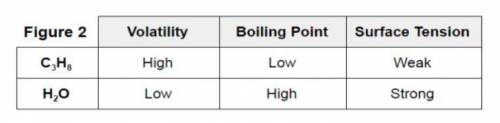
Consider the information provided in experiment #3 Figure 2. Three different variables are compared for C3H8 (propane) and H2O (water). The strength of the intermolecular forces are responsible for the behavior of the two compounds.
Identify the intermolecular force for the two compounds.
Which one is stronger?
Explain how the strength of intermolecular forces plays a role in at least two of the properties (volatility, boiling point, or surface tension) for C3H8 (propane) and H2O (water).
Cite evidence from the data to explain your reasoning.
Experiment #3: The student follows study #2 with a comparison of propane (C3H8) and water. Volatility is the tendency for a substance to vaporize and it was found that propane vaporizes quickly while water vaporized slowly. Surface tension is a physical property equal to the amount of force per unit area necessary to expand the surface of a liquid. It is this phenomenon which allows a water strider or lizard to run across the surface of water.
100 points

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3
You know the right answer?
Consider the information provided in experiment #3 Figure 2. Three different variables are compared...
Questions

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00

Mathematics, 07.07.2019 17:00


History, 07.07.2019 17:00


Mathematics, 07.07.2019 17:00


English, 07.07.2019 17:00




Mathematics, 07.07.2019 17:00




Social Studies, 07.07.2019 17:00

Social Studies, 07.07.2019 17:00



