
Chemistry, 16.10.2020 18:01 Clover1072
A student performs a redox titration to determine the percent by mass of chlorate in an allergy tablet. The chlorate is titrated with iodide ions until the end point. The student reported that 29.50 mL of 0.100 M KI solution was required to reach the end point of a titration when 10 allergy tablets containing chlorate as the main active ingredient are dissolved in 25.00 mL of distilled water. What mass in grams of chlorate is present in the 10 allergy tablets?
Use the Balanced chemical equation to answer the question.
6, 1, 6, 3, 1, 3
Hint: this is a net ionic equation, so spectator ions have been removed!
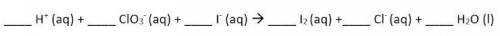

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
A student performs a redox titration to determine the percent by mass of chlorate in an allergy tabl...
Questions




Mathematics, 11.03.2021 16:10

History, 11.03.2021 16:10

Mathematics, 11.03.2021 16:10

Physics, 11.03.2021 16:10

Mathematics, 11.03.2021 16:10



History, 11.03.2021 16:10

Mathematics, 11.03.2021 16:10

History, 11.03.2021 16:10

Social Studies, 11.03.2021 16:10



History, 11.03.2021 16:10


Mathematics, 11.03.2021 16:10

Business, 11.03.2021 16:10



