
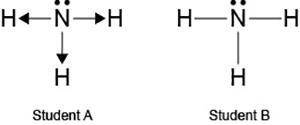
Two students made the Lewis dot diagrams of NH3. The diagrams are as shown.
Two visual diagrams of an N H three molecule are shown. Student As diagram on the left has nitrogen at the center and connects with each hydrogen atom by an arrow pointing away from the nitrogen, one below, one on the right, and one on the left. There is a pair of dots above the nitrogen atom. Student Bs diagram on the right has nitrogen at the center connecting by a straight line to each hydrogen positioned below, to the left, and to the right of nitrogen. There is a pair of dots above the nitrogen atom.
Which student drew the correct Lewis dot diagram? (4 points)
Only Student A
Only Student B
Both Student A and Student B
Neither Student A nor Student B

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Two students made the Lewis dot diagrams of NH3. The diagrams are as shown.
Two visual diagrams of...
Questions

Social Studies, 05.05.2020 22:10

Spanish, 05.05.2020 22:10


Social Studies, 05.05.2020 22:10


Mathematics, 05.05.2020 22:10




Mathematics, 05.05.2020 22:10

Mathematics, 05.05.2020 22:10


Computers and Technology, 05.05.2020 22:10



History, 05.05.2020 22:10




History, 05.05.2020 22:10



