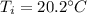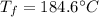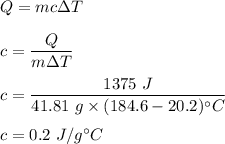Chemistry, 16.10.2020 15:01 tegaoks6843
A 41.81 g sample of a substance is initially at 20.2 °C. After absorbing 1375 J of heat, the temperature of the substance is 184.6 °C. What is the specific heat (c) of the substance?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
A 41.81 g sample of a substance is initially at 20.2 °C. After absorbing 1375 J of heat, the tempera...
Questions

Mathematics, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40

World Languages, 20.04.2021 16:40




Mathematics, 20.04.2021 16:40


Mathematics, 20.04.2021 16:40

Biology, 20.04.2021 16:40

English, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40


Mathematics, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40

Mathematics, 20.04.2021 16:40

Social Studies, 20.04.2021 16:40