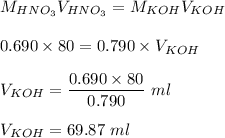Chemistry, 16.10.2020 15:01 graciemccain
A volume of 80.0 mL of a 0.690 M HNO3 solution is titrated with 0.790 M KOH. Calculate the volume of KOH required to reach the equivalence point. Express your answer to three significant figures and include the appropriate units.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
A volume of 80.0 mL of a 0.690 M HNO3 solution is titrated with 0.790 M KOH. Calculate the volume of...
Questions

Mathematics, 04.03.2021 08:00


Mathematics, 04.03.2021 08:00


Health, 04.03.2021 08:00


Mathematics, 04.03.2021 08:00

Mathematics, 04.03.2021 08:00


Social Studies, 04.03.2021 08:00


Mathematics, 04.03.2021 08:00


Mathematics, 04.03.2021 08:00

Mathematics, 04.03.2021 08:00





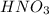 solution is titrated with 0.790 M KOH.
solution is titrated with 0.790 M KOH.