
Chemistry, 16.10.2020 20:01 kcarstensen59070
Ionization energy is the energy required to remove an electron from an atom. Which atom from the list below would be the most difficult to remove an electron from?
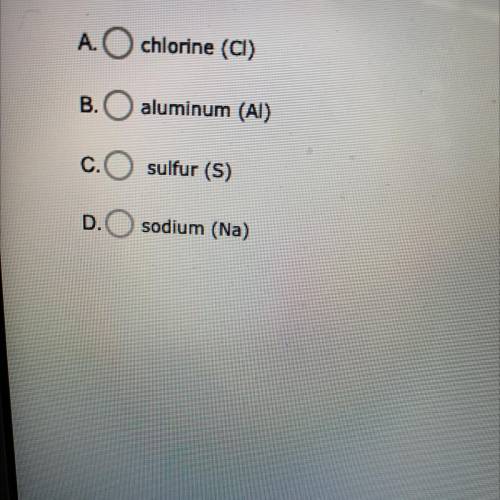

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Ionization energy is the energy required to remove an electron from an atom. Which atom from the lis...
Questions

English, 28.06.2021 06:10

Chemistry, 28.06.2021 06:10

Mathematics, 28.06.2021 06:10

Social Studies, 28.06.2021 06:20

Mathematics, 28.06.2021 06:20

Mathematics, 28.06.2021 06:20


English, 28.06.2021 06:20

Mathematics, 28.06.2021 06:20



Mathematics, 28.06.2021 06:20

Mathematics, 28.06.2021 06:20

Social Studies, 28.06.2021 06:20

Mathematics, 28.06.2021 06:20


Mathematics, 28.06.2021 06:20

English, 28.06.2021 06:20


Health, 28.06.2021 06:20



