

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

You know the right answer?
For the chemical reaction
2AgNO3+Na2CrO4⟶Ag2CrO4+2NaNO3
what mass of silver chromate i...
what mass of silver chromate i...
Questions

Mathematics, 18.10.2019 07:00





Mathematics, 18.10.2019 07:00

Mathematics, 18.10.2019 07:00


Business, 18.10.2019 07:00



Mathematics, 18.10.2019 07:00



Spanish, 18.10.2019 07:00



History, 18.10.2019 07:00

Social Studies, 18.10.2019 07:00

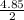 = 2.43moles of Ag₂CrO₄
= 2.43moles of Ag₂CrO₄ 

