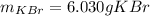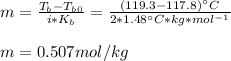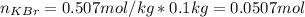Chemistry, 17.10.2020 14:01 sconner733
A certain liquid has a normal boiling point of and a boiling point elevation constant . A solution is prepared by dissolving some potassium bromide () in of . This solution boils at . Calculate the mass of that was dissolved.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
A certain liquid has a normal boiling point of and a boiling point elevation constant . A solution i...
Questions

Chemistry, 10.12.2019 09:31

Mathematics, 10.12.2019 09:31



Social Studies, 10.12.2019 09:31

Social Studies, 10.12.2019 09:31

English, 10.12.2019 09:31



Mathematics, 10.12.2019 09:31

English, 10.12.2019 09:31




Mathematics, 10.12.2019 09:31

Mathematics, 10.12.2019 09:31


Computers and Technology, 10.12.2019 09:31