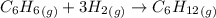The initial pressure of a mixture of C6H6 and an excess of H2 in a rigid vessel is 1.21 atm. A catalyst is introduced. After the reaction reaches completion, the temperature is restored to its initial value. The final pressure in the vessel is 0.839 atm. What was the mole fraction of C6H6 in the original mixture

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
The initial pressure of a mixture of C6H6 and an excess of H2 in a rigid vessel is 1.21 atm. A catal...
Questions

Physics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00


Mathematics, 21.11.2020 01:00

English, 21.11.2020 01:00

Biology, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00



Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Arts, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

English, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00