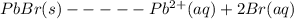Chemistry, 17.10.2020 16:01 ilovemeatballs5
Determine the value of the equilibrium constant (report your answer to three significant figures) for the following reaction if an equilibrium mixture contains 0.010 mol of solid PbBr2, and is 0.0100 M in Pb2+ ions and 0.0250 M in Br1- ions. Use the notation 4.31e-5 to indicate a number such as 4.31 x 10-5.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Determine the value of the equilibrium constant (report your answer to three significant figures) fo...
Questions

Mathematics, 16.01.2021 14:00


Mathematics, 16.01.2021 14:00

Biology, 16.01.2021 14:00

Mathematics, 16.01.2021 14:00

English, 16.01.2021 14:00

Mathematics, 16.01.2021 14:00

Spanish, 16.01.2021 14:00

Biology, 16.01.2021 14:00


Mathematics, 16.01.2021 14:00

Mathematics, 16.01.2021 14:00



Mathematics, 16.01.2021 14:00

Mathematics, 16.01.2021 14:00




Mathematics, 16.01.2021 14:00