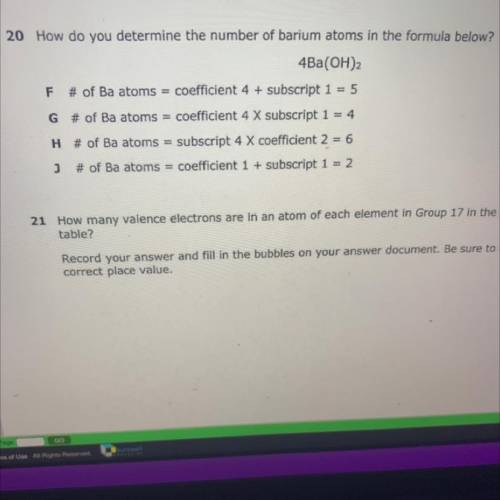
G is correct option:
# of Ba atoms = coefficient 4 × subscript 1= 4
Explanation:
Formula:
4Ba(OH)₂
G is correct option:
# of Ba atoms = coefficient 4 × subscript 1= 4
Because there are only 4 atoms of Ba in given formula.
Ba(OH)₂ contain one atom of Ba while in question there are 4 moles of Ba(OH)₂ present thus total 4×1 = 4 atoms of Ba present.
Other options are incorrect. Because,
F:
# of Ba atoms = coefficient 4 + subscript 1 = 5
This shows given formula contain 5 Ba atoms. So it is incorrect.
H:
# of Ba atoms = subscript 4 × coefficient 2 = 6
This shows that subscript is 4 which is incorrect because coefficient is 4 and subscript is 1.
j:
# of Ba atoms = subscript 1 + coefficient 1 = 2
This option shows that subscript is one which is correct but coefficient is incorrect. The coefficient of Ba is 4 and coefficient is always multiply with subscript not added. So this option is also incorrect.