
Chemistry, 18.10.2020 15:01 lucaszsanches
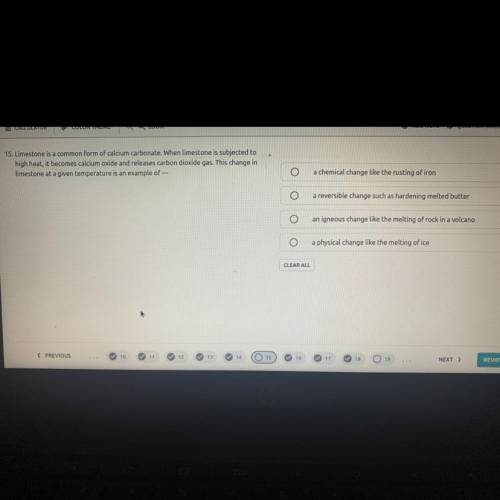
Limestone is a common form of calcium carbonate. When limestone is subjected to
high heat, it becomes calcium oxide and releases carbon dioxide gas. This change in
Limestone at a given temperature is an example of ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3


Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Limestone is a common form of calcium carbonate. When limestone is subjected to
high heat, it becom...
Questions

English, 21.06.2019 17:50



Mathematics, 21.06.2019 18:00



Chemistry, 21.06.2019 18:00


English, 21.06.2019 18:00

Mathematics, 21.06.2019 18:00

World Languages, 21.06.2019 18:00


Mathematics, 21.06.2019 18:00

Mathematics, 21.06.2019 18:00

Mathematics, 21.06.2019 18:00

Spanish, 21.06.2019 18:00

Mathematics, 21.06.2019 18:00

Mathematics, 21.06.2019 18:00





