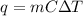Chemistry, 18.10.2020 15:01 bikerhomie
Problem:
Determine the amount of energy (heat) in Joules and Kcal required to raise the temperature 0f 98.5 grams water from 37.0 0C to 75.0 0C.
Restate the problem (in your own words & all relevant information included).
Model that best represents the problem. Draw and label.
Steps to solve the problem (include formula, known and unknown variables, etc)
Solution and final answer. (show cancellation of units).
Reflection: Must at least have 3 sentences.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
Problem:
Determine the amount of energy (heat) in Joules and Kcal required to raise the temperature...
Questions

History, 29.10.2020 16:40


Mathematics, 29.10.2020 16:40

Advanced Placement (AP), 29.10.2020 16:40


Mathematics, 29.10.2020 16:40


Mathematics, 29.10.2020 16:40

History, 29.10.2020 16:40

Mathematics, 29.10.2020 16:40

Mathematics, 29.10.2020 16:40


Mathematics, 29.10.2020 16:40



Mathematics, 29.10.2020 16:40

Mathematics, 29.10.2020 16:40

English, 29.10.2020 16:40