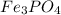Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:10
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be processed to obtain 1.5 kg of pb?
Answers: 1


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
A 13.58 g sample of a compound contains 8.67 g of iron, Fe, 1.60 g of phosphorus, P, and oxygen, O....
Questions



Mathematics, 22.10.2021 20:20


History, 22.10.2021 20:20

Mathematics, 22.10.2021 20:20



Advanced Placement (AP), 22.10.2021 20:20


Mathematics, 22.10.2021 20:20

Physics, 22.10.2021 20:20




Biology, 22.10.2021 20:20

Social Studies, 22.10.2021 20:20


History, 22.10.2021 20:20