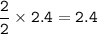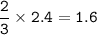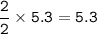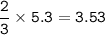Chemistry, 19.10.2020 04:01 mosesbrinker
23. For the reaction shown, calculate how many moles of each
product form when the given amount of each reactant com-
pletely reacts. Assume there is more than enough of the
other reactant.
2PbS(s) + 302(g) → 2PbO(s) + 2S02(8)
(a) 2.4 mol PbS
(b) 2.4 mol O2
(c) 5.3 mol PbS
(d) 5.3 mol O2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

You know the right answer?
23. For the reaction shown, calculate how many moles of each
product form when the given amount of...
Questions






Mathematics, 20.02.2020 19:31

Mathematics, 20.02.2020 19:31



Computers and Technology, 20.02.2020 19:31






English, 20.02.2020 19:31


Mathematics, 20.02.2020 19:31