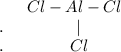Chemistry, 19.10.2020 22:01 anthony3913
When drawing the Lewis structure of a molecule, start by determining the total number of available valence based on each element's group number. Then, use the total number of electrons needed for each element to be stable, generally based on its charge, to determine the ionic charge by finding the difference between the number of needed and available electrons divided by two. Next, identify the central atom, which is the element with the fewest valence electrons other than hydrogen. Finally, arrange the number of bonds around the central atom to fulfill the stable number of electrons for each element.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1


Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

You know the right answer?
When drawing the Lewis structure of a molecule, start by determining the total number of available v...
Questions

Mathematics, 13.02.2021 23:20

Mathematics, 13.02.2021 23:20



Mathematics, 13.02.2021 23:20



Mathematics, 13.02.2021 23:20





History, 13.02.2021 23:20


Mathematics, 13.02.2021 23:20


Arts, 13.02.2021 23:20