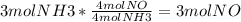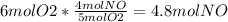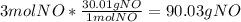Chemistry, 20.10.2020 07:01 inucornspineapple
3. What is the Limiting Reactant in the following equation if you start with 3 moles of NH3 with 6 moles of O2?
4NH3 (g) + 5O2 (g) 4NO (g) + 6H2O(g)
4. How many grams of NO can be made from the previous equation and quantities?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1
You know the right answer?
3. What is the Limiting Reactant in the following equation if you start with 3 moles of NH3 with 6 m...
Questions




English, 18.07.2019 13:00

English, 18.07.2019 13:00



Mathematics, 18.07.2019 13:00






Social Studies, 18.07.2019 13:00

Physics, 18.07.2019 13:00

Mathematics, 18.07.2019 13:00


Chemistry, 18.07.2019 13:00

History, 18.07.2019 13:00