
Chemistry, 20.10.2020 16:01 Flameking1223
Aspirin is a weak organic acid whose molecular formula is HC9H7O4. An aqueous solution of aspirin is prepared by dissolving 3.60 g/L. The pH of this solution is found to be 2.6. Calculate Ka for aspirin. (atomic mass: C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3

Chemistry, 23.06.2019 13:30
Asap what is the temperature when the volume is 700 ml? a 500 k b 200 k c 600 k d 700 k
Answers: 1
You know the right answer?
Aspirin is a weak organic acid whose molecular formula is HC9H7O4. An aqueous solution of aspirin is...
Questions

Mathematics, 20.09.2021 14:00

Mathematics, 20.09.2021 14:00

Mathematics, 20.09.2021 14:00

Mathematics, 20.09.2021 14:00





History, 20.09.2021 14:00



Mathematics, 20.09.2021 14:00

Mathematics, 20.09.2021 14:00

History, 20.09.2021 14:00

History, 20.09.2021 14:00




Mathematics, 20.09.2021 14:00

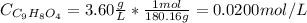
![Ka = \frac{[C_{9}H_{7}O_{4}^{-}][H_{3}O^{+}]}{[C_{9}H_{8}O_{4}]}](/tpl/images/0824/0933/a1a8e.png)
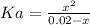
![pH = -log[H_{3}O^{+}]](/tpl/images/0824/0933/b7638.png)
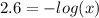
![x = 2.51 \cdot 10^{-3} M = [H_{3}O^{+}] = [C_{9}H_{7}O_{4}^{-}]](/tpl/images/0824/0933/98904.png)
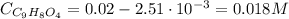
![Ka = \frac{[C_{9}H_{7}O_{4}^{-}][H_{3}O^{+}]}{[C_{9}H_{8}O_{4}]} = \frac{(2.51 \cdot 10^{-3})^{2}}{0.018} = 3.50 \cdot 10^{-4}](/tpl/images/0824/0933/393ec.png)



