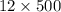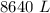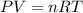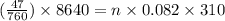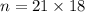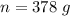Chemistry, 20.10.2020 17:01 aiken11192006
Assume that the inspired air is dry air. The vapor pressure of 47 mmHg at 37°C corresponds to a volume and mass of liquid water. If the tidal volume is 500 mL and respiration rate is 12 min−1, how much water is lost from the body per day? Would you expect this to increase or decrease with activity?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3



Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2
You know the right answer?
Assume that the inspired air is dry air. The vapor pressure of 47 mmHg at 37°C corresponds to a volu...
Questions

History, 30.07.2019 14:00


Arts, 30.07.2019 14:00

Social Studies, 30.07.2019 14:00

History, 30.07.2019 14:00

History, 30.07.2019 14:00

Biology, 30.07.2019 14:00

Social Studies, 30.07.2019 14:00

Mathematics, 30.07.2019 14:00

History, 30.07.2019 14:00

History, 30.07.2019 14:00

History, 30.07.2019 14:00

History, 30.07.2019 14:00


Geography, 30.07.2019 14:00

Chemistry, 30.07.2019 14:00



Social Studies, 30.07.2019 14:00

History, 30.07.2019 14:00