Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1


Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
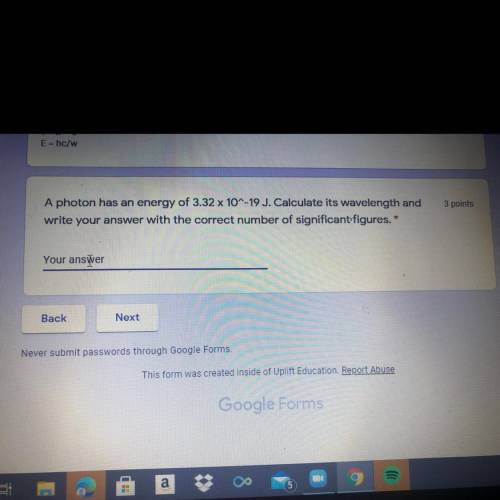
A photon has an energy of 3.32 x 10^-19 J. Calculate its wavelength and
write your answer with the...
Questions

Biology, 19.09.2019 10:30




Mathematics, 19.09.2019 10:30

Mathematics, 19.09.2019 10:30


Chemistry, 19.09.2019 10:30

History, 19.09.2019 10:30

Social Studies, 19.09.2019 10:30




Physics, 19.09.2019 10:30


History, 19.09.2019 10:30


Biology, 19.09.2019 10:30

Mathematics, 19.09.2019 10:30

English, 19.09.2019 10:30