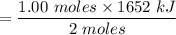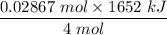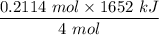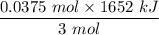Chemistry, 20.10.2020 20:01 channarlawassociate
The overall reaction in a commercial heat pack can be represented as How much heat is released when 4.40 moles of iron are reacted with excess ? Heat = kJ How much heat is released when 1.00 mole of is produced? Heat = kJ How much heat is released when 1.60 g iron is reacted with excess ? Heat = kJ How much heat is released when 11.8 g and 1.20 g are reacted? Heat = kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1


Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
The overall reaction in a commercial heat pack can be represented as How much heat is released when...
Questions

Geography, 13.05.2021 02:50


Social Studies, 13.05.2021 02:50

History, 13.05.2021 02:50

Mathematics, 13.05.2021 02:50


Mathematics, 13.05.2021 02:50


Mathematics, 13.05.2021 02:50

Mathematics, 13.05.2021 02:50

Mathematics, 13.05.2021 02:50

Biology, 13.05.2021 02:50


English, 13.05.2021 02:50


Mathematics, 13.05.2021 02:50


Mathematics, 13.05.2021 02:50

Mathematics, 13.05.2021 02:50

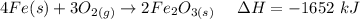
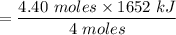
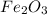 is produced?
is produced?